

Amsino has acquired Canadian based manufacturer MedXL. This acquisition will expand Amsino’s existing North American manufacturing and expand existing prefilled syringe capacity to more than 500 million syringes annually.
MedXL has been committed to providing medical devices to pharmaceutical, consumer, and healthcare communities for over 25 years. Blending industry-advanced technology with a team approach to quality, they offer the industry’s most comprehensive advantage, encompassing medical devices.
All of MedXL’s facilities are FDA and Health Canada registered, as well as ISO and MDSAP certified for regulatory compliance.

Amsino has acquired Canadian based manufacturer MedXL. This acquisition will expand Amsino’s existing North American manufacturing and expand existing prefilled syringe capacity to more than 500 million syringes annually.
MedXL has been committed to providing medical devices to pharmaceutical, consumer, and healthcare communities for over 25 years. Blending industry-advanced technology with a team approach to quality, they offer the industry’s most comprehensive advantage, encompassing medical devices.
All of MedXL’s facilities are FDA and Health Canada registered, as well as ISO and MDSAP certified for regulatory compliance.
“The acquisition aligns perfectly with our reshoring strategy to expand our manufacturing footprint to better serve customers in North America. Given today's geopolitical environment and the uncertainties the industry is facing, onshore manufacturing increasingly aligns with many of our customers’ supply chain resiliency strategies.”
- Dr. Richard Lee, Founder, Chairman, and CEO of Amsino.
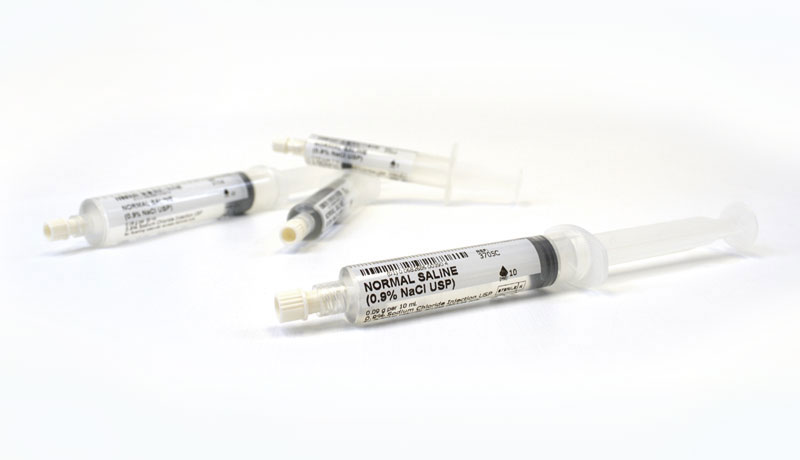
Manufactured in Canada according to ISO Standards, current Good Manufacturing Practices and MedXL Quality Control requirements, MedXL saline prefilled syringes set the standard for quality and safety. These syringes are terminally sterilized and can be used in a sterile field. Saline prefilled syringes are latex and preservative free. MedXL saline prefilled syringes are secure, reliable and accurate.
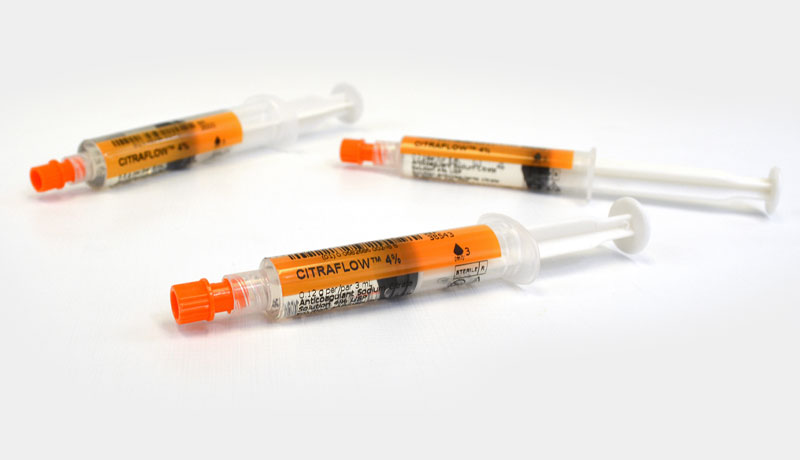
CitraFlow™ prefilled syringes are a proven alternative to heparin for locking indwelling catheters on patients. Sodium citrate avoids the risks associated with systemic heparinization and is safe for use in patients with suspected or confirmed Heparin Induced Thrombocytopenia.
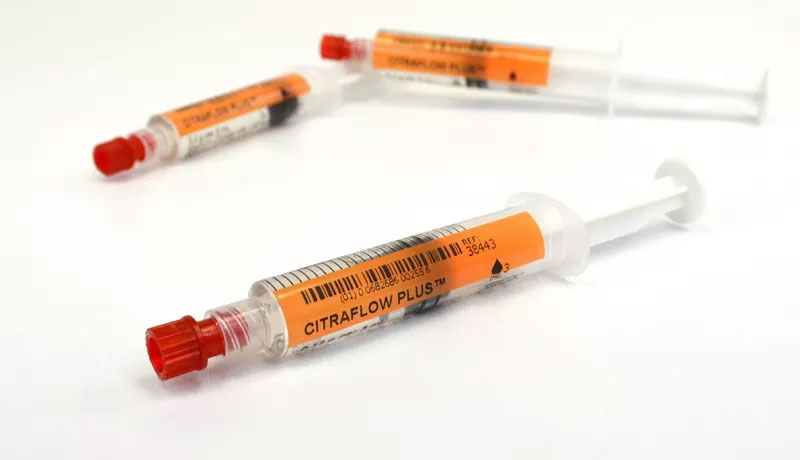
CitraFlow PLUS™ prefilled syringes combines the anticoagulant properties of sodium citrate PLUS the antimicrobial properties of ethanol in one syringe. This solution combination prevents biofilm formation, reduces incidences of CRBSI (catheter related blood stream infections) as well as being a safe alternative to heparin.
Interested in learning more about our new prefilled syringe offerings? Share your contact information below and our team will reach out to you with more information.